We are developing a unique approach that uses affordable IR cameras (e.g., the $249 FLIR ONE) to visualize invisible energy flows and transformations in easy-to-do science experiments. Using this "desktop remote sensing" approach, thermal energy can be readily "seen." Other types of energy that convert into thermal energy can be inferred from thermal signals. Hence, many invisible physical, chemical, and biological processes that absorb or release heat can be visualized, discovered, and investigated.
Experiments
Comparing the Heats of Solution of Table Salt and Baking Soda
This video shows a comparison of heats of solution of baking soda and table salt. A teaspoon of salt and a teaspoon of baking soda
were added to two cups of water, respectively.
Read more »
« Link to this
Vapor Pressure Lowering: Experiment One
This IR video shows that the evaporation rate of water from saltwater is smaller than that from freshwater.
This is deduced from the weakening of the evaporative cooling effect in the saltwater container.
Read more »
« Link to this
Vapor Pressure Lowering: Experiment Two
This IR video shows that the evaporation rate of water from a cup of saltwater is smaller than that from a cup of freshwater.
This is deduced from the weakening of the condensation heating effect onto a piece of paper above the saltwater cup.
Read more »
« Link to this
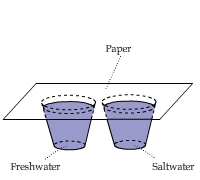
Paper Hung over Freshwater & Saltwater
Two paper strips were hung above a cup of saltwater and a cup of freshwater, respectively. The paper strips touched the water.
This video shows that the paper area above the water line in the freshwater cup was about 7°C cooler than room temperature. Above
the saltwater, this cooling effect was much less significant.
Read more »
« Link to this
Paper Towel Hung over Freshwater & Saltwater
Two strips of paper towel were hung above a cup of saltwater and a cup of freshwater, respectively. The strips touched the water.
The cooling effect on the strip above the saltwater was less significant, indicating the diffusion of salt ions into the paper
towel to weaken water evaporation.
Read more »
« Link to this
Why does Convection Stop in Saltwater?
An ice cube was put into a cup of freshwater and saltwater, respectively. This IR video shows that the melting of the ice cube was slower
in saltwater than in freshwater.
Read more »
« Link to this
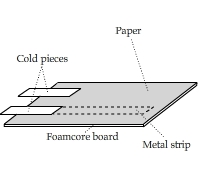
Why do Metals Feel Colder?
This video shows a comparison of thermal conductivities of metals and foams. A thin metal sheet was placed on top of a foamcore board and
was covered by a piece of paper. The thumbs were used as identical heaters.
Read more »
« Link to this
Do Metals Always Feel Colder?
It depends. In a very hot day when the ambient temperature exceeds body temperature, a piece of metal feels warmer than a piece of foam,
even though they are both at the same temperature. This experiment simulates that situation by using two identical cold objects taken out from
a freezer and placing them on top of a metal piece and foam.
Read more »
« Link to this
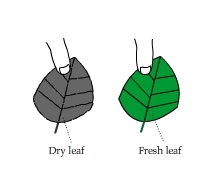
A Dry Leaf vs. a Fresh Leaf
One way to tell if an indoor plant is a plastic or a real one is to touch its leaves. Have you wondered why real leaves feel cooler?
This experiment compares the heat patterns of a dry leaf and a fresh leaf from the same plant.
Read more »
« Link to this
Why do Leaves Feel Cooler?
One way to tell if an indoor plant is a plastic or a real one is to touch its leaves. Have you wondered why real leaves feel cooler?
They are not cooler — they cannot be seen through an IR camera, meaning that they are at ambient temperature. The
key is the water stored in their sponge layers. This experiment used a dry and a wet sponge to show this.
Read more »
« Link to this