We are developing a unique approach that uses affordable IR cameras (e.g., FLIR ONE) to visualize invisible energy flows and transformations in easy-to-do science experiments. Using this approach, thermal energy can be readily "seen." Other types of energy that convert into thermal energy can be inferred from thermal signals. Hence, many invisible physical, chemical, and biological processes that absorb or release heat can be visualized, discovered, and investigated, paving the road to the development of a universal thermal indicator of any such process.
Experiments
Evaporation, Condensation, & Equilibrium
Put a piece of paper above a cup of water.
The water appeared to be cooler than room temperature because of evaporative cooling. The paper somehow recovered
the energy lost through evaporation and warmed up dramatically. How did this happen?
Read more »
« Link to this
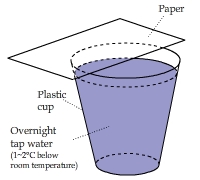
Four Temperature Zones on a Piece of Paper
A piece of cardstock was placed above a cup of water for a couple of hours. Then it was shifted a bit. Can you explain the
pattern that quickly emerged?
Read more »
« Link to this
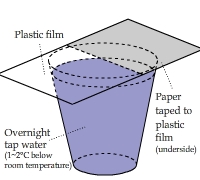
Absorbing Water Molecules: Paper vs. Plastic
A piece of paper was taped onto half of a sheet of plastic film and then put the sheet on top of a cup of water
(with the paper underside).
The result showed significant condensation onto the paper but nothing onto the plastic surface.
Read more »
« Link to this
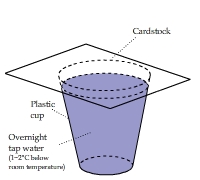
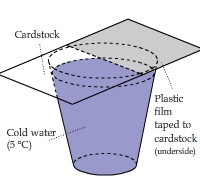
Cardstock and Plastic above Cold Water
A piece of cardstock, half of which was covered by a sheet of plastic film, was placed on top of a cup of cold water.
The cardstock and plastic film above the water quickly cooled off. There was no condensation heating effect as shown in the above two clips.
Read more »
« Link to this
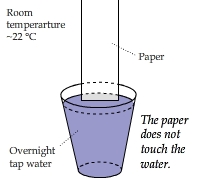
What Warms Up the Paper?
A piece of paper approached (but not touched) the surface of water from the perpendicular direction
and was warmed up. It appeared as if heat were transferred from cool water to the paper. How is this possible?
Read more »
« Link to this
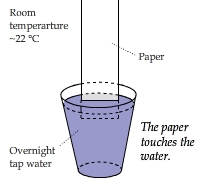
Capillary Action on Paper
Water molecules diffuse through a paper strip hung above a cup of water (and touching it) through capillary action.
This video shows the temperature distribution of the paper. The coolest area on the paper was just above the water line,
where the capillary action was the most obvious.
Read more »
« Link to this
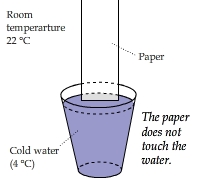
Hanging a Piece of Paper above Cold Water
A piece of paper approached (but not touched) the surface of cold water from the perpendicular direction and was cooled down.
Read more »
« Link to this
The Formation of Bénard-Marangoni Convection Cells in Cooking Oil
This video reveals the formation of a "cellular" thermal pattern in heated cooking oil.
Read more »
« Link to this
The Stability of Bénard-Marangoni Convection Cells in Cooking Oil
This video reveals a pretty stable cellular thermal pattern in heated cooking oil. Shaking the frying pan a few times didn't seem to break the pattern.
Read more »
« Link to this
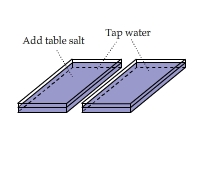
Visualizing the Heat of Solution of Table Salt
There are two containers side by side. Add a couple of spoon of table salt to one of them. Stir to accelerate the dissolving of salt
and compare the IR views of the two containers.
Read more »
« Link to this